Green hydrogen is moving beyond pilot scale to commercials involving gigawatts of electricity, so the electrolyzers for hydrogen production are now a vital boardroom question and not a technical engineering measurement. The International Energy Agency represents hydrogen as a key to decarbonization of the hard-to-abate sectors, including steel production, ammonia creation of ammonia, chemical production, and transportation. However, the cost-competitiveness of hydrogen powered by fossil fuels solely depends on the ability to increase the efficiency of electrolyzers to definite levels.
The principle of best hydrogen electrolyzer efficiency is wider than an industrial scale. Even small systems like 3000 ml lab-scale or wellness-scale units illustrate why efficiency is a concern of highly significant importance. A high-efficiency hydrogen generator electrolyser with an electrical efficiency of 65-70% (or equivalent of 56 kWh/kg) is radically different from inefficient designs with an electrical efficiency of 70-75 kWh/kg. In case of constant small-scale operation of its integrated application over the course of thousands of hours each year, these differences add up significantly, with real consequences to the overall cost of ownership and long-run sustainability of operation. The market has become aware of this need: a company that can boast of 41.5 kwh/kg system-level performance is now worth a premium, whereas designs that were left behind at 52-55 kwh/kg are experiencing an increasingly exerted pressure on their margins.
This is a complete guide to understand the operation of the electrolyzers used to produce hydrogen, the reasons why efficiency is the main point of distinction, and how stakeholders ought to compare them with other systems to find the most suitable hydrogen generator electrolyzer to use in their respective operations.

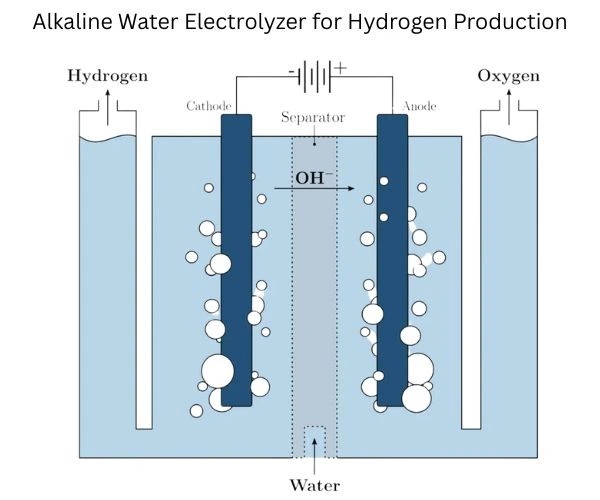
How does hydrogen electrolyzer work? Water electrolysis is an illusion of a very simple idea that conceals great engineering complexity. The basic chemistry is simple: by direct electrical feeds, H 2O was separated into hydrogen and oxygen. However, to convert this graceful chemistry into effective, long-lasting, low-cost systems, complex engineering in many and many integrity where energy tradeoffs are increasing is the case.
The essential process works in the following important steps:
The thermodynamic level provides physical limits that are absolute. The theoretical minimum is 39.4 kWh/kg H 2, which is the enthalpy of hydrogen burning. This has been the physical lower limit that can never be reduced. The reality systems always surpass this by natural entropy rise in the process of water disaggregation and thermodynamic irreversibility. The practical lower theoretical limit of consideration of legitimate thermodynamic constraints is approximated to be 39.4-40 kWh/kg under standard conditions.
Recent business systems differ widely: alkaline systems use 50-60 kWh/kg; PEM systems use 50-55 kWh/kg; advanced SOEC systems can use 37.5 kWh/kg with high-temperature processes in which the thermal energy replaces the electrical energy to a great extent.
It is important to know the meaning of stack and system efficiency:
The rising curve of efficiency is hastened. Design targets of Current PEM: 65 percent (LHV) by 2026 and 72 percent. This corresponds to a decrease of 55 kWh/kg currently to 49 kWh/kg by 2026- it proves that drastic progress is still feasible and cost-effective.
Levelized Cost of Hydrogen (LCOH) formula: efficiency has an economic leverage:
Efficiency has an influence on three out of four LCOH components:
To start with, the efficiency directly affects the CAPEX amortization. Increased efficiency translates to increased hydrogen per unit capacity and decreased cost of capital on each kilogram. An electrolyzer of 50 kW, having a 55 kwh/kg, esterifies 909 kg/year (8000 hrs of operation). The same system produces 1,111 kg/year at 45 kWh/kg, which is a 22 percent increase of output, and the extra capital required is none. This has a tremendous impact of minimizing 1/kg CAPEX expenditure.
Second, the greatest cost of operation is the electricity cost. At a cost of 0.05/kWh (average in facilities powered by renewable energy):
With a 1 MWe hydrogen electrolyzer efficiency operating at 90 capacity with a yearly output of 78,840 kg /kg, the improvement of 10 kWh /kg would result in a saving of $528, 600/year, or $10.6 million over 20 years. This is a reduction by more than 20 percent of cost alone due to efficiency at 3/kg LCOH.
Third, there are significant savings in the cost of O&M. Reduced electricity throughput results in less cooling needs, less water usage, and less severe conditions in which components operate, increasing service life. Alkaline stacks reach 60,000 hours and more, PEM 40,000 -80,000 hours.
The economics of the hydrogen electrolyzer price paradox seem to portray conflicting economics. Stack implementation in high-efficiency is more expensive. The design necessitates that channel-feds be precise. Emerging platinum-group catalyst membranes are more expensive. However, total lifecycle cost would prefer systems with a high level of efficiency since the savings in electricity are extremely large compared to the CAPEX premiums.
There are three mature technologies of electrolyser that control the market with unique efficiency features and deployment conditions:
In the case of industrial hydrogen electrolyzer purchasers (utilities, corporations, hydrogen developers) the purchasing model is an equilibrium between conflicting requirements:
The hydrogen electrolyzer price (initial CAPEX) should be judiciously compared with predicted savings through the lifecycle. A 20 percent CAPEM estimate is tolerable provided that it saves 10 percent in lifecycle expense by enhancing efficiency. The highest operation cost is the price of electricity which is driven by hydrogen electrolyzer efficiency. System footprint influences the cost of installation and the complexity to run. Vendor track record: established experience of operation, warranty provision, availability of spare parts, and so on have an impact on the risk assessment.
With smaller users (labs, clinics, wellness centers), the choice of a hydrogen generator electrolyzer will be different:
Flow rate and response time should meet the operational needs. Specifications of purity differ radically: a fuel cell system needs H 2 of higher than 99999 percent purity; analytical standards are smaller. Maintenance is paramount–laboratory technicians are uncertain of power systems knowledge; a plug-and-play power system with limited maintenance structures is expensive but demands minimal maintenance premiums. Cumulative ownership cost, including consumables (membranes, electrolyte) and water usage, maintenance expenses, and electricity are of greater significance than initial cost.
Part of warranties and service support is also vital. Electrolyzers are complicated devices- direct costs involve downtime. Hydrogen electrolyzer manufacturers with extended warranty (510 years), guaranteed response service (24-hour service by a technician) attract a 10-15% premium. This support is particularly valued by smaller laboratories and wellness centers because they do not possess much technical expertise.
The efficiency of hydrogen electrolyzers is improved – It is the most significant part of green hydrogen, and in this way, it can be economically viable and without damaging the climate. The existing systems with 50-60 kWh/kg should be enhanced to 40-45 kWh/kg in order to compete with fossil-based hydrogen. The 41.5 kWh/kg capillary-fed technology developed by Hysata and the SOEC of 37.5 kWh/kg developed by Bloom prove to be very dramatic but technically possible.
Knowledge of the operation of a hydrogen electrolyzer, the many steps involved in conditioning the water up to the separation of gases, shows that efficiency is gained through methodical optimization of all the parts. The distinctions of the key varieties of hydrogen electrolyzers display staggering importance: in scenarios that are sensitive to cost, alkaline takes the lead, in those that require integration with renewable energy, PEM is more likely to succeed, and in those that demand heat use, SOEC comes out.
To the stakeholders attempting to assess the electrolyzers for hydrogen production, investment choices should focus on the lifecycle-based pricing rather than the initial cost. The 15 percent efficiency over 20 years will save millions per MW, justifying the extra upfront investment easily. The lowest-priced best hydrogen electrolyzer is not necessarily the most desirable: one that offers the lowest total cost of ownership and provides reliability as well as facilitates changes of technology quickly.
The businesses that reach the 41.5kWh/kg and the rivals that remain at the 52-55kWh/kg are constructing the hydrogen economy, driving the global decarbonization. Efficiency is not a luxury; it is the existential dictum of what investments prosper, and the other category of investment becomes stranded.
Hydrogen future is the one who achieves the art of hydrogen electrolyzer efficiency.